Shells and Bonding
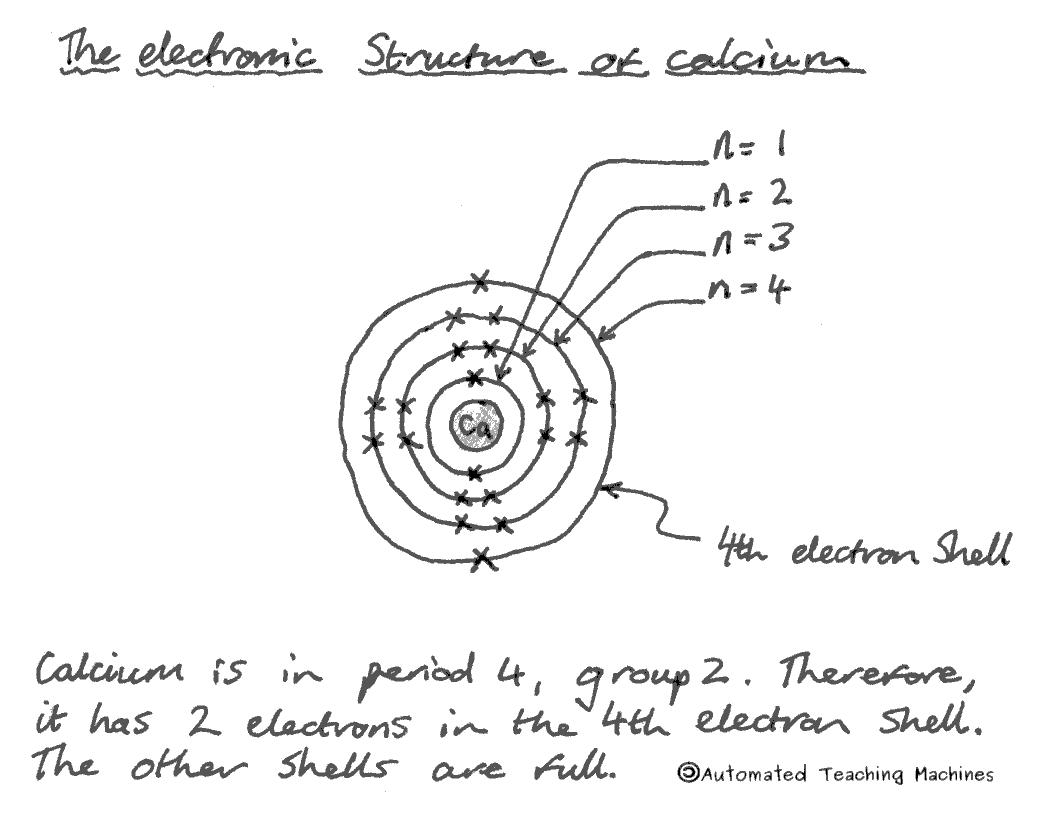
The electronic structure of calcium is 2,8,8,2. This tells us how many electrons are in each shell. The number of electron shells in an element is the same as its period number in the periodic table.
During chemical reactions, elements in group 1 lose electrons, whereas elements in group 7 gain electrons. They do this to achieve the state of having a full outer shell. An atom which has either lost or gained electrons is called an ion.
In group 1, atoms that are larger are more reactive because it is easier for them to lose an electron. However, in group 7, larger atoms are less reactive because it is harder for them to attract electrons.