Rates of Reaction
Scientists may want to control the rate of a reaction to make it safer or to be able to produce a substance quickly and cheaply.
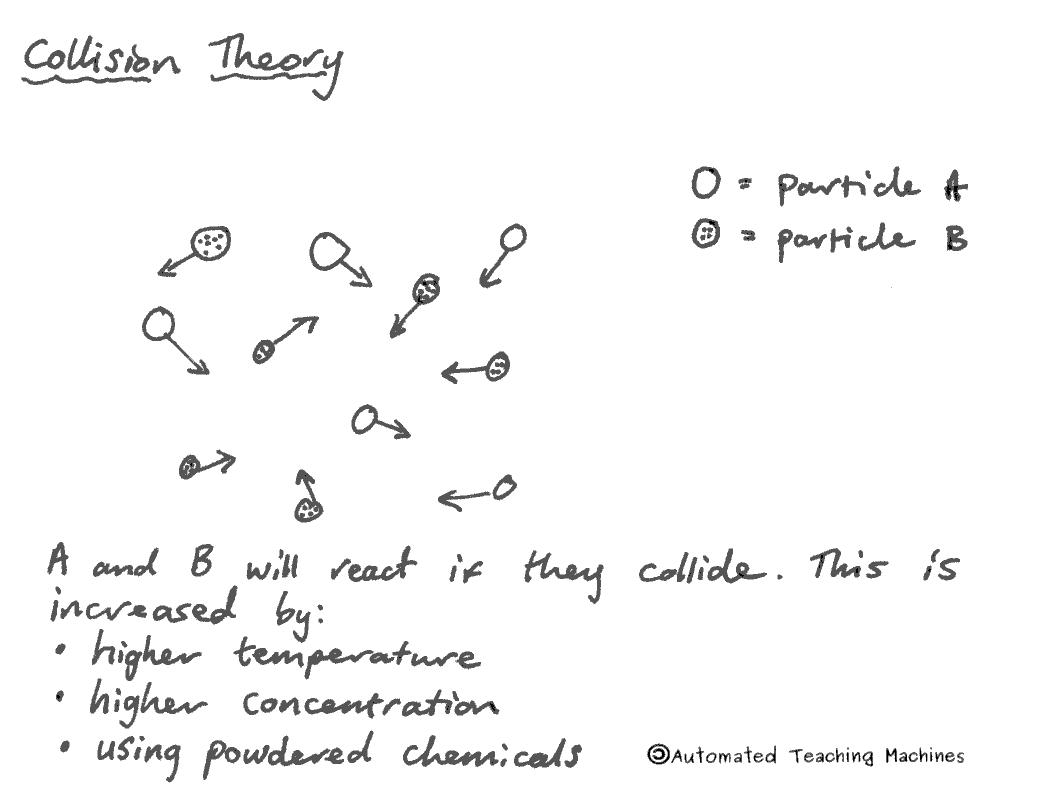
We can speed up chemical reactions by making them hotter, using more concentrated solutions, using powdered chemicals, or by adding a substance called a catalyst, that will speed up the reaction without being affected itself. The first three methods increase the collision frequency between the reactants.
The rate of a reaction is a measure of the amount of change in the chemicals per second. This can be determined by measuring the change in mass, volume of gas given off, or the change in colour or transparency over time.