Metals and Alloys
Metal atoms are held together by strong metallic bonds so they are usually strong, malleable and have high melting points. They have free electrons which makes them good conductors of electricity and heat.
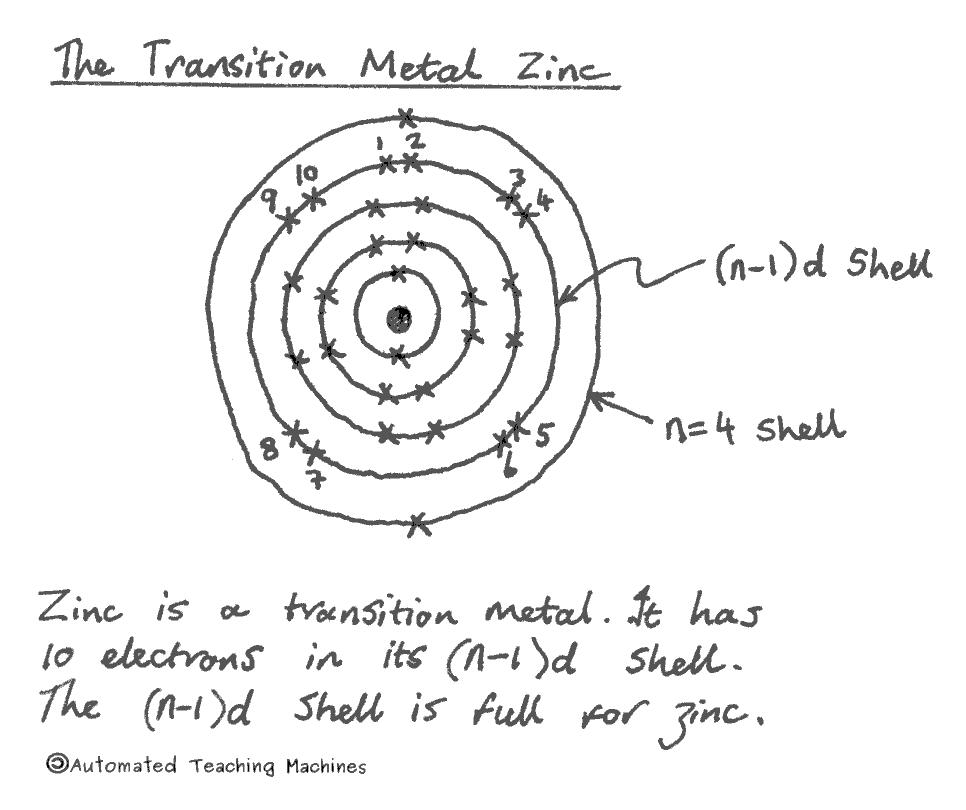
Most metals, including iron, copper, zinc and gold are transition metals which are found in the middle section of the periodic table and have electrons in the (n-1)d shell which can hold up to ten electrons. This is why this 'd-block' is ten elements wide.
Alloys are mixtures of a metal with another metal or other elements. Brass is an alloy of copper and zinc, 18 carat gold is an alloy of gold and copper and steel is an alloy of iron and carbon.