Metal Extraction
Metals that are less reactive than carbon can be extracted by heating the powdered metal ore with carbon in the form of coke. Carbon removes oxygen from iron oxide in a blast furnace to produce iron and carbon dioxide. The ore is reduced because it loses oxygen and the carbon is oxidised.
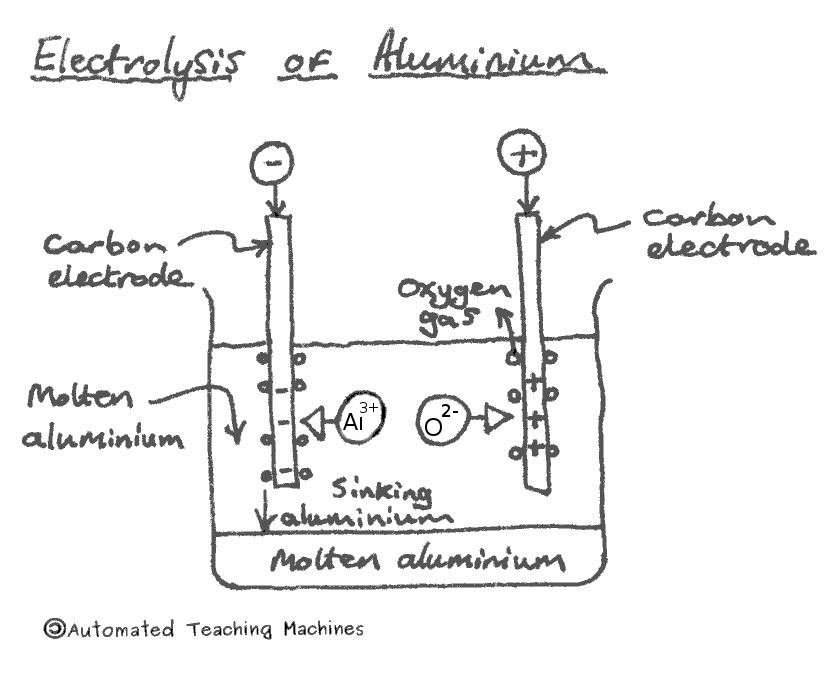
More reactive metals, like aluminium, can be extracted by electrolysis. Electricity is passed through molten aluminium oxide and positive aluminium ions move towards the negative electrode, gain electrons and form molten aluminium metal which sinks to the bottom of the tank. Oxygen is produced at the positive electrode where oxygen ions lose electrons.